
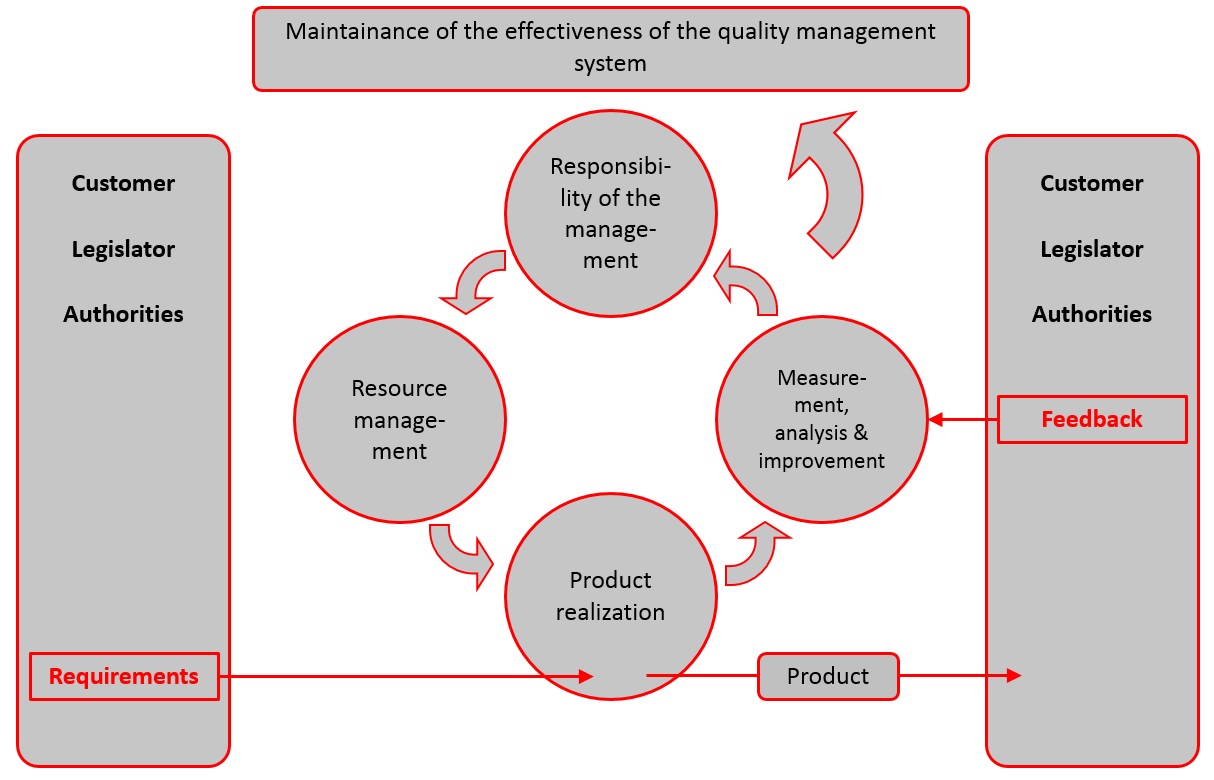
Product Realization: Covers everything from planning and designing to the process’s quality control.Resource Management: Provides requirements for resources such as personnel and training courses.Management Responsibility: Includes the requirements for management’s role in the QMS, including management review, policies, objectives, and customer satisfaction efforts.Quality Management System: Requirements for the overall quality management system documentation, including document control, requirement procedures, and forms control.Terms and Definitions: Provides definitions and frames the terminology used throughout the standard.Normative References: Provides the fundamentals, common vocabulary, and other references for quality management systems.Scope: Describes the purpose of the standard and its use by organizations and manufacturers.
#En iso 13485 iso#
(If your lab does seek accreditation, it must pass a third-party Medical Device Single Audit Program or “MDSAP” audit.)īelow is an overview of the ISO 13485 requirements: While it’s not mandatory, a third-party certification demonstrates your commitment to the safety and quality of medical devices. A medical device manufacturing lab is accredited only after satisfying the applicable regulatory requirements of ISO standards and demonstrating a commitment to medical device quality and safety. The ISO 13485 certification process is a multistep approach. (You can also create your own QMS, as long as it meets the legal and regulatory requirements for manufactured and sellable medical devices.) The implementation of the ISO 13485 standard, while recommended, is not mandatory. How is the ISO 13485 Accreditation Structured?

The accreditation bolsters credibility and overall trust in a manufacturer’s processes and end-products.

Global recognition and better alignment with the Food and Drug Administration (FDA): ISO 13485 is recognized by the International Medical Device Regulators Forum and is the model QMS standard in major worldwide markets and medical industries.Some of the other benefits of ISO 13485 standards are mentioned below: The implementation of ISO 13485 provides a practical foundation for medical device manufacturers it allows manufacturers to meet the Medical Device Directives (in EU and UK), regulations, and responsibilities for the safety and quality of medical devices. The ISO 13485 standard goes hand-in-hand with ISO 9001, with the main difference being ISO 13485’s added layer of customer satisfaction and continuous improvements. It’s also used by internal and external parties, such as certification bodies, to assist with their auditing processes. ISO 13485 applies to medical device industries and manufacturers (those involved in the design, production, installation, and servicing of medical devices and any related services). In this case, a medical device is defined as a product (ranging from larger machines to in vitro reagents to individual tools) that is intended for use in the prevention, diagnosis, and treatment of diseases or other medical conditions. It’s one of the most comprehensive standards for medical device manufacturers to use in order to meet customer and applicable QMS regulatory requirements. Quality management system ISO 13485 is an international and very commonly used QMS regulatory standard for medical devices.


 0 kommentar(er)
0 kommentar(er)
